The Lucira Check It COVID-19 Test Kit is a diagnostic test intended to detect the novel coronavirus SARS-COV-2 that causes COVID-19. The test is intended to detect if you have an active infection and does not confirm immunity or detect antibodies. It is a molecular test that amplifies the virus’s genetic material while the test is running just like PCR lab tests. Lucira’s amplification method provides a level of accuracy comparable to one of the highest sensitivity lab PCR tests.
In two Community Testing Studies which included individuals with and without COVID-19 symptoms, the Lucira test was compared to the Hologic Panther Fusion, one of the highest sensitivity FDA authorized SARS-CoV-2 PCR tests. Lucira achieved 98% accuracy, 97% positive percent agreement (PPA) and 98% negative percent agreement (NPA)(1), excluding samples with very low levels of virus that possibly no longer reflected active infection (2). Across all data, and including 10 samples with very low viral loads (>37.5 Ct), Lucira achieved 96% accuracy, 92% PPA, and 98% NPA (3).
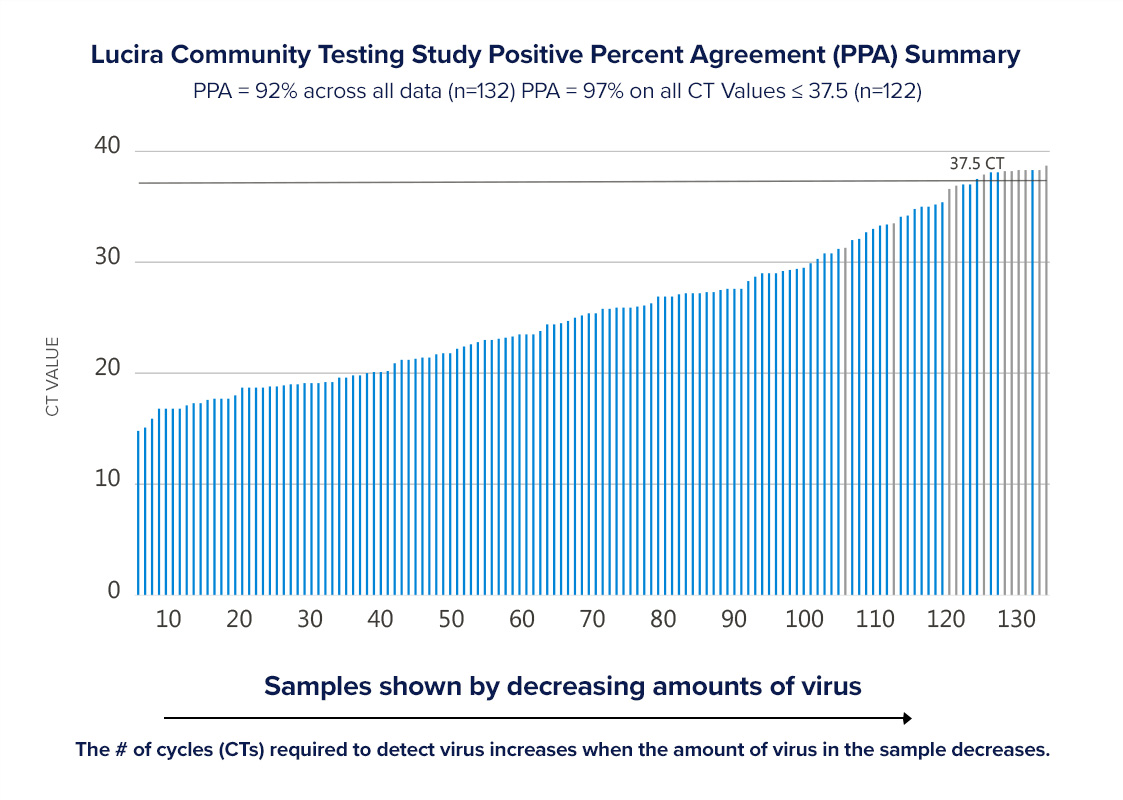
This graph shows the Lucira Check It COVID-19 Test Kit positive percent agreement in Lucira’s two Community Testing Studies. BLUE bars represent samples where the Lucira positive test result matched the comparison test result. GREY bars represent the Lucira test results that were negative and did not match the comparison test positive result. Nearly all of the GREY bars occurred in samples where there were very low levels of virus detected by the comparison test.
However, it is still possible that this test can give a false result. Test results should always be considered in the context of clinical observations and epidemiological data (such as local prevalence rates and current outbreak/epicenter locations) in making a final diagnosis and patient management decisions.
(1) Lucira Community Testing Study 07A-CLI 006 Fall 2020 and Lucira Community Testing Study 07A-CLI 007 Winter 2021 (n=394)
(2) La Scola B., Clinical Infectious Diseases, September 2020
(3) Lucira Community Testing Study 07A-CLI 006 Fall 2020 and Lucira Community Testing Study 07A-CLI 007 Winter 2021 (n=404)